-40%
TWO BURDICK CADIO VIVE AED WITH SOFTWARE ONE MANUAL + GOOD BATTERIES + BOX
$ 278.78
- Description
- Size Guide
Description
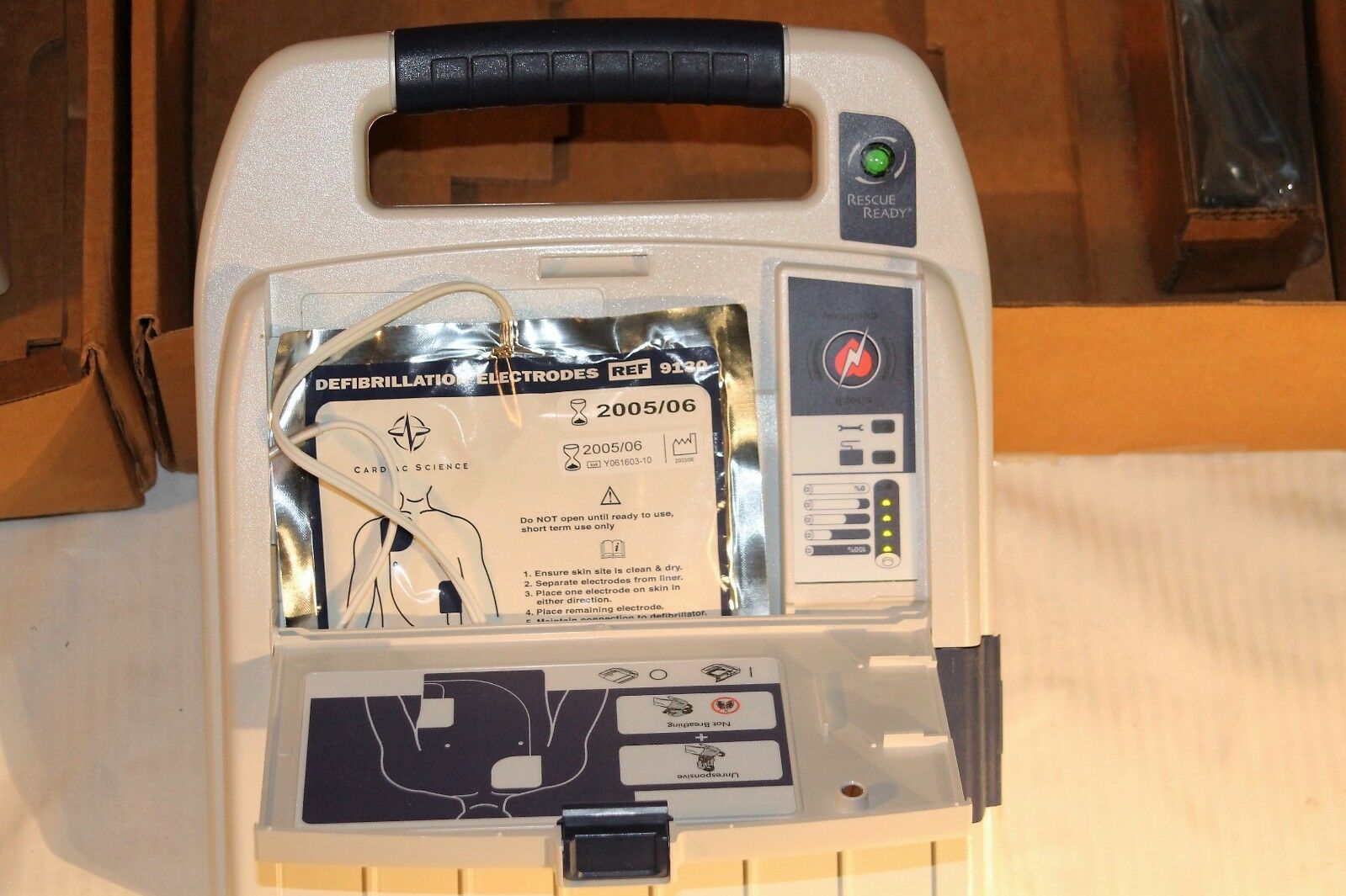
LOT OF 2 BURDICK CADIO VIVE AED WITH SOFTWARE ONE MANUAL + GOOD BATTERIES + BOXThis listing is for a LOT OF 2 UNITS
Burdick Cardio Vive AUTOMATED EXTERNAL DEFIBRILLATOR AED
Model Number 92530
Serial Number 92530-0000255
It may need a new battery. Or charger if this is a rechargeable battery. The battery is branded Cardiac Science on is available on online auctions.
The item powers up the LED's come goes through some kind of self test. And an audible message says Battery low place electrodes on patient’s chest
It includes the
• Burdick Cardiovive
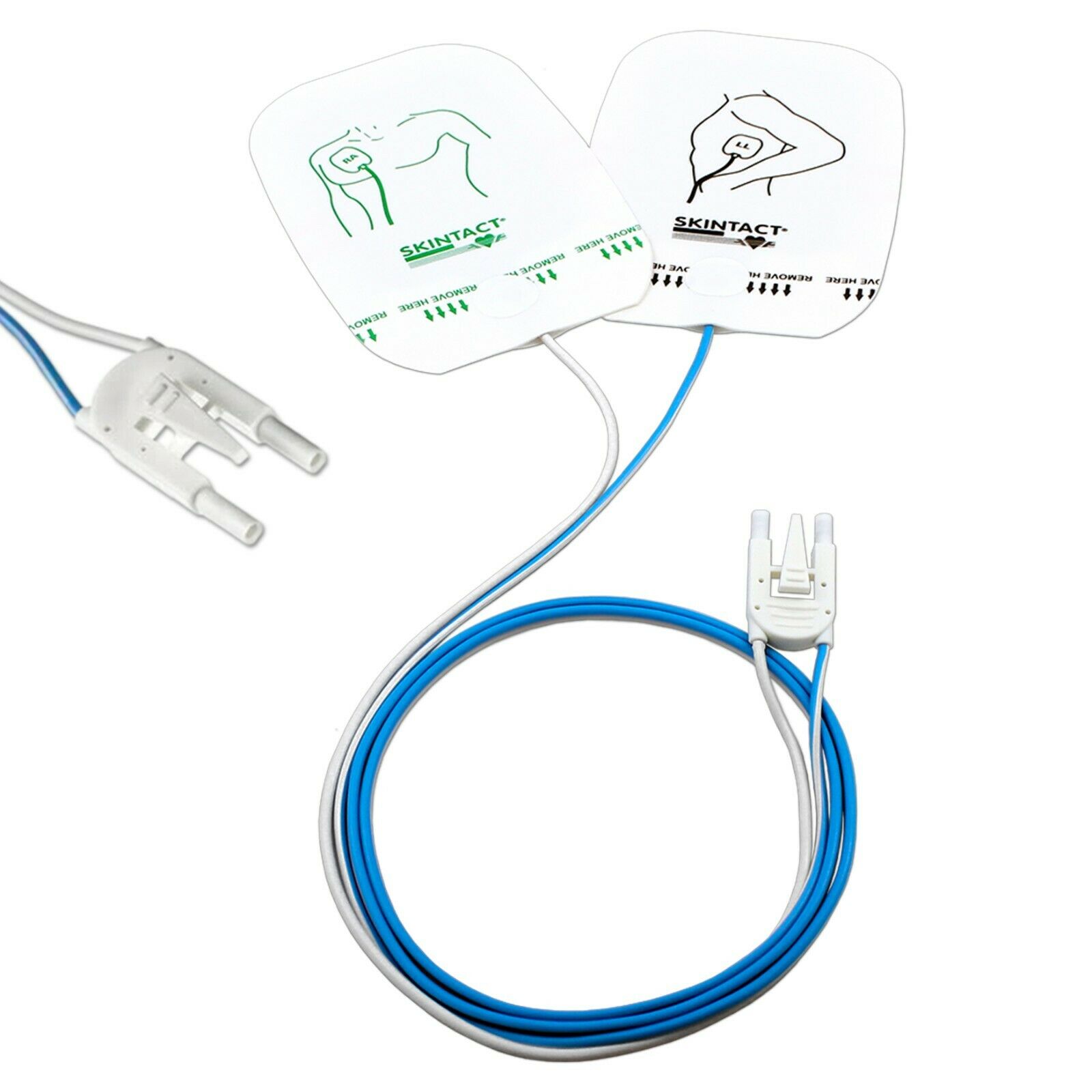
• A sealed set of Electrodes
• A Battery with an exp date 2005 / 2006
• The Operating Instructions / Training Book
• A data cable
• RescueLink Version 8.3 on CD
The boxes are worn
Except for the power up these have not been tested and are sold as is.
They are in good cosmetic condition.
Bidders should be familiar with this item.
we do not know how to fully test this item. (
BOTH unit ARE showing the RESCUE READY indicator
)
We are not in the Medical Equipment Business. Sometimes other equipment is included in lots we buy.
Most of the time we are only able to perform basic test (power up/ self test)
Except for the power up test this item is untested and sold as is.
The listing is for the items listed only no other chords, cable's software or manuals.
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, only bid if you are an authorized purchaser. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item."
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health: